
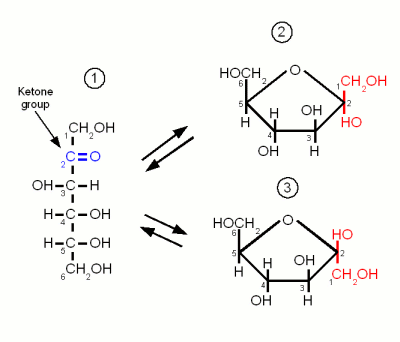
Two monosaccharides with equivalent molecular graphs (same chain length and same carbonyl position) may still be distinct stereoisomers, whose molecules differ in spatial orientation. So, for example, H(C=O)(CHOH) 4H is pentose, H(CHOH)(C=O)(CHOH) 3H is pentulose, and H(CHOH) 2(C=O)(CHOH) 2H is pent-3-ulose. In the latter case, if the carbonyl is not at position 2, its position is then indicated by a numeric infix.
The various classifications above can be combined, resulting in names such as "aldohexose" and "ketotriose".Ī more general nomenclature for open-chain monosaccharides combines a Greek prefix to indicate the number of carbons (tri-, tetr-, pent-, hex-, etc.) with the suffixes "-ose" for aldoses and "-ulose" for ketoses. Ketoses of biological interest usually have the carbonyl at position 2. Otherwise, the molecule has a ketone group, a carbonyl −(C=O)− between two carbons then it is formally a ketone, and is termed a ketose. In that case, the compound is termed an aldose. If the carbonyl is at position 1 (that is, n or m is zero), the molecule begins with a formyl group H(C=O)− and is technically an aldehyde.

Monosaccharides are the simplest units of carbohydrates and the simplest form of sugar. Therefore, the molecular structure of a simple monosaccharide can be written as H(CHOH) n(C=O)(CHOH) mH, where n + 1 + m = x so that its elemental formula is C xH 2 xO x.īy convention, the carbon atoms are numbered from 1 to x along the backbone, starting from the end that is closest to the C=O group. Simple monosaccharides have a linear and unbranched carbon skeleton with one carbonyl (C=O) functional group, and one hydroxyl (OH) group on each of the remaining carbon atoms. In aqueous solutions monosaccharides exist as rings if they have more than four carbons. Monosaccharides with eight or more carbons are rarely observed as they are quite unstable. Examples of heptoses include the ketoses, mannoheptulose and sedoheptulose. Ribose and deoxyribose (in RNA and DNA, respectively) are pentose sugars. Glucose, used as an energy source and for the synthesis of starch, glycogen and cellulose, is a hexose. Monosaccharides can be classified by the number x of carbon atoms they contain: triose (3), tetrose (4), pentose (5), hexose (6), heptose (7), and so on. With few exceptions (e.g., deoxyribose), monosaccharides have this chemical formula: (CH 2O) x, where conventionally x ≥ 3. The monosaccharide glucose plays a pivotal role in metabolism, where the chemical energy is extracted through glycolysis and the citric acid cycle to provide energy to living organisms. For instance, galactose and glucose are both aldohexoses, but have different physical structures and chemical properties. This gives rise to a number of isomeric forms, all with the same chemical formula. Įach carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). Most monosaccharides have the formula (CH 2O) (though not all molecules with this formula are monosaccharides).Įxamples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Contrary to their name (sugars), only some monosaccharides have a sweet taste. They are usually colorless, water- soluble, and crystalline solids. Monosaccharides (from Greek monos: single, sacchar: sugar), also called simple sugars, are the simplest forms of sugar and the most basic units ( monomers) from which all carbohydrates are built. ( Learn how and when to remove this template message) Please improve this article to make it neutral in tone and meet Wikipedia's quality standards.


 0 kommentar(er)
0 kommentar(er)
